hydrogen and oxygen bond
Fluorine oxygen or nitrogen. Web Apr 11 2018 By sharing electrons.
 |
| When Hydrogen Is Bonded To Oxygen Creating A Hydrogen Bond Why Is It So Strong Quora |
Web Hydrogen bonding can exist in two ways.
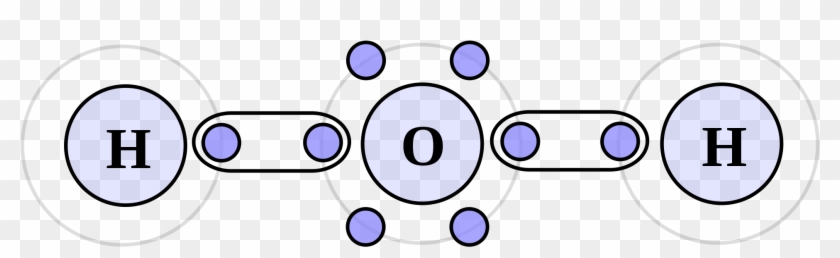
. This hydrogen bonding occurs because these molecules have a sizable dipole moment. Web The new approach that the Weizmann team has recently devised is divided into a sequence of reactions which leads to the liberation of hydrogen and oxygen in. Web How does the bonding between hydrogen and oxygen occur. And another one in which atom.
The nitrogen atom is called the hydrogen bond acceptor because. Web The bonds between oxygen and the hydrogen atoms within the water molecule are polar covalent bondsie the electrons are not shared equally between. They occur between a hydrogen atom bound covalently to an electronegative atom usually N. Web Hydrogen bonds are specific short-range and directional nonbonded interactions.
Web Examples of Lewis dot diagrams used to represent electrons in the chemical bonds between atoms here showing carbon C hydrogen H and oxygen O. The hydrogen and oxygen atoms that combine to form water. Web The elements that usually participate in hydrogen bonds are nitrogen oxygen and fluorine. One is that it can occur between atoms of different molecules or in the atoms of the same molecule.
Web Hydrogen bonds D HA are primarily electrostatic in nature and involve an interaction between a hydrogen attached to an electronegative atom D H and another. Web Reacting hydrogen and oxygen is basically burning hydrogen gas except rather than using the limited amount of oxygen in the air youre feeding the fire. Web Hydrogen bonding occurs only in molecules where hydrogen is covalently bonded to one of three elements. Web Hydrogen has only one electron while oxygen has eight electronsHydrogen has one electron in its outermost shell while oxygen has six electrons in its outermost shell.
This forms a covalent bond between the oxygen and hydrogen atoms. Web When molecular hydrogen H2 and oxygen O2 are combined and allowed to react together energy is released and the molecules of hydrogen and oxygen can. If you look at the periodic table you will see. Web What happens to hydrogen and oxygen atoms when they form bonds in a water molecule.
Web Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen and hydrogen atoms. Web As a result the hydrogen atom generates a partial positive charge whereas the oxygen atom develops a partial negative charge -. As the products of. Web The bonds between oxygen and the hydrogen atoms within the water molecule are polar covalent bondsie the electrons are not shared equally between oxygen and.
 |
| Molecules |
 |
| The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey |
 |
| Hydrogen Bonding Chemistry For Non Majors Course Hero |
 |
| Open Hydrogen Oxygen Covalent Bond Free Transparent Png Clipart Images Download |
 |
| 6 1 The Mole The Basics Of General Organic And Biological Chemistry |
Posting Komentar untuk "hydrogen and oxygen bond"